Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.
With the rampant spread of SARS - coronavirus 2 responsible for the recent pandemic COVID 19, we have heard the WHO time and again advise us to wash our hands with soap. Not just water, but soap. Why is that you may wonder.
If you think about it, we often resort to washing our skin with soap when something oily sticks to it, like when we get grease on our hands or when our face turns sticky. Soap comes to great use when those sticky little oil molecules grab onto our skin, and water just doesn’t seem to help in washing it away.
In principle, soap helps kill the coronavirus in the exact same way. Let’s explore how, shall we?
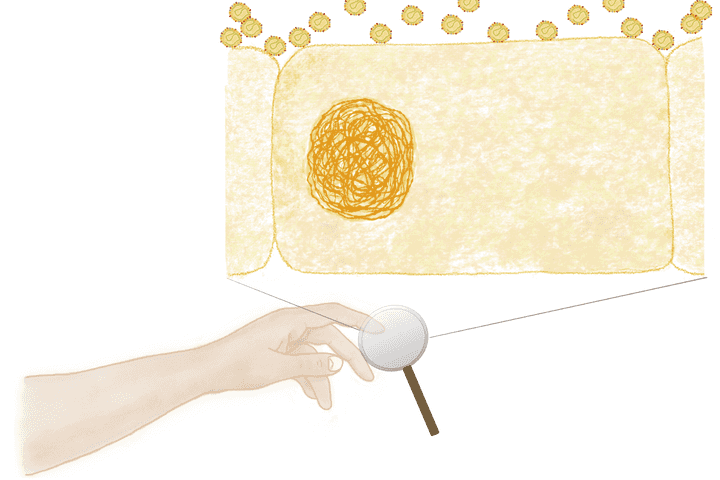
coronavirus particles settling on the cells of the skin. © Sunaina Rao.
An infected COVID 19 individual, releases thousands of virus-containing droplets each time he/she coughs or sneezes. Each droplet harbours millions of coronavirus particles. These particles once in the air, will eventually settle on the nearest surface. On touching this surface, the viruses latch onto your hand, and from here they readily hitch a ride into your nasal tract, where an active infection can begin. So, what’s of utmost importance is to not only wash away these viruses as soon as possible, but also destroy them in the process.
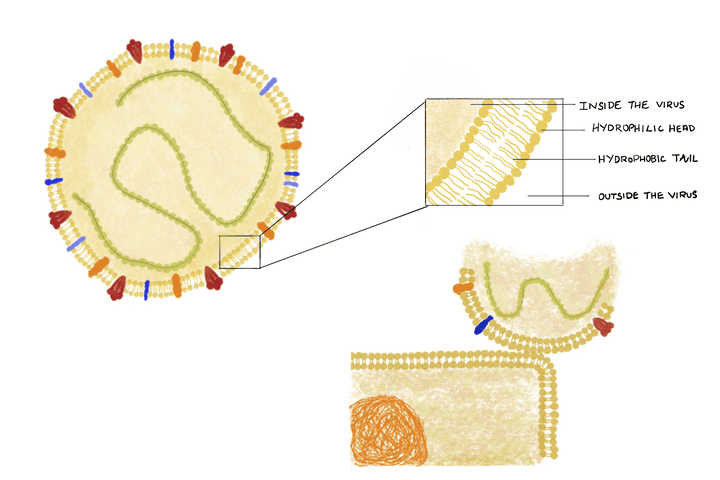
Structure of the coronavirus particle and its interaction with a skin cell. © Sunaina Rao.
Now before we dwell into how we can destroy a virus particle; we first need to understand its structure. The coronavirus, or any other virus for that matter has three main biochemical components:
What’s note worthy here is that human cells, including the ones on the skin, have a similar outer lipid bi layer. This similarity makes it easier for the virus to interact with the cell and stick onto it (Refer image).
Structure of soap molecules. © Sunaina Rao.
Doctors and researchers have widely recommended a good wash of the hands with soap, as a simple yet effective way of killing any coronavirus on the hand. A virus with such a high level of infectivity, killed by a simple bar of soap? The reason behind this is the chemistry of the soap. Soap is largely made of molecules that are salts of fatty acids, with a hydrophilic head group and hydrophobic tails (Refer image). Hence their structure is very similar to the lipids in the lipid bi-layer of the viruses and human cells.
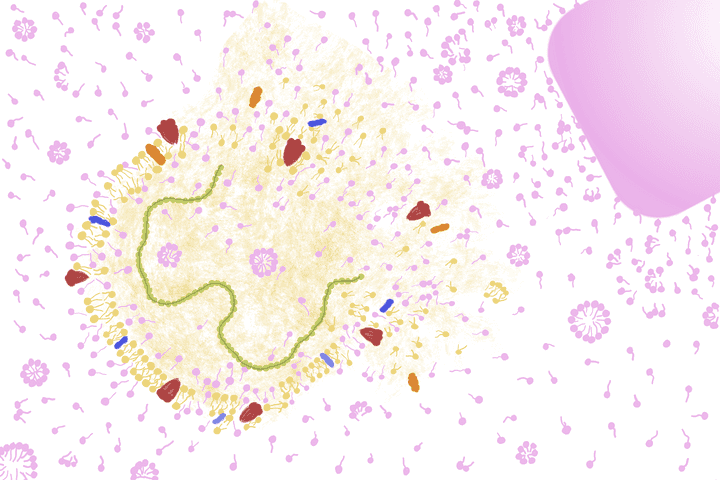
The soap molecules compete with the lipids in the viral lipid bi-layer, disassembling the entire framework of the virus. © Sunaina Rao.
When soap is mixed with water, the soap molecules get dispersed in the water. This solution acts in two ways.
Firstly, since its structure is so similar to that of the lipid molecules in the lipid bi-layer, it competes with the viruses to bind to the skin, there by dislodging the viruses.
Secondly, again owing to its structure, it competes with the lipids in viral lipid bi-layer. Remember how the lipids in the bi-layer are held together by weak interactions? Due to this, the soap molecule manages to easily insert itself into the layer, disrupting the viral outer case. In the process, all the other molecules part of this intricate structure like the proteins and RNA, also fall apart, crumbling the very framework of the virus.
Now that we know the science behind how soap works, it wouldn’t hurt to spend that extra 20 seconds to rub our hands with some frothy soap solution, would it?