Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.
1981 brought to light an unusually dreadful disease. A disease that had a surprisingly higher incidence in homosexual men, causing lethal infections and rare cancers. The relentless research on this disease that ensued in the following years showed that these men were almost defenceless against infectious agents. Their T cells (white blood cells), the front runners of the immune system, were found to be sharply diminishing day by day. They were severely immunocompromised. With nearly 38 million people across the world living with this infection, we now call this disease AIDS (Acquired Immune Deficiency Syndrome) and the vicious virus that causes it all - HIV (Human Immunodeficiency Virus). The pressing question, however, is why scientists have not been successful in preventing this infection?
The COVID-19 pandemic (2021) has been an eye-opener when it comes to the incredible utility of vaccines. The first two years of the pandemic gave us 8 WHO-approved vaccines, many of them eliciting over 90 percent efficacy in preventing hospitalisation and death. Yet, 40 years and 100’s of clinical trials later, we still have no vaccine for HIV. In fact, the highest efficacy against the infection obtained by any vaccine candidate so far is a modest 31 percent (The Thai vaccine trial) (Rerks-Ngarm et al., 2009). Though this might appear as a serious scientific failure, a closer look reveals the immensely complex nature of HIV itself and the terrific advancements made by scientists in devising several vaccine candidates despite this complexity.
But why is the HIV vaccine proving so difficult to design and what are some of the promising vaccines being tested at the moment? - Let’s explore!
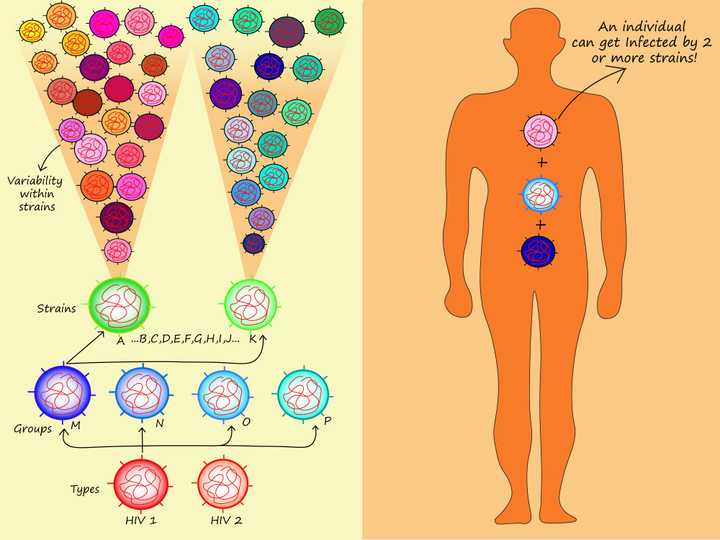
Left: HIV and its different strains. Right: An individual can get infected by 2 or more strains at the same time. © Sunaina Rao
The biggest obstacle (Ng’uni et al., 2020) in developing an HIV vaccine is the astonishingly high rate at which HIV can mutate. Imagine a con artist, disguising himself in clever ways to commit his world-class crimes. HIV could certainly give a tight competition to the best con artist out there!
The outer surface of HIV is embedded with proteins called the envelope (Env) proteins (comparable to the spike proteins of the SARS-CoV-2 that we have come to be familiar with). These proteins specifically recognise T cells and a few other cells of the immune system. Once HIV has made contact with its target cell, it enters inside it and uses distinct enzymes to make hundreds of copies of itself and bursts out, killing the target cell in the process.
However, interestingly, one of its enzymes, called the reverse transcriptase, does a very poor job of making copies of the viral genetic material. It incorporates several ‘errors’ or mutations in HIV’s genes. This means that each virus particle that comes bursting out of the target cell is considerably different from the parent virus that went in. In fact, HIV mutates up to 10 times per round of replication in a target cell. The newborn viruses continue infecting other cells, mutating once again when they replicate.
Although shoddy work can be largely useless in our ‘human’ world, in the world of HIV, the unbelievable shoddiness of the reverse transcriptase gives HIV a superior advantage. The mutations in the HIV genes create a vast diversity of HIV envelope proteins. Now, these are the very proteins against which our body makes antibodies. Hence HIV outwits the immune system, as antibody production lags behind a fast mutating virus. Adding to the complexity is the fact that these viruses infect the very cells that are involved in antibody production. Are you also beginning to appreciate the ingenuity of this virus? The scientists working on HIV vaccine design certainly are!
A vaccine, in essence, consists of a mixture of viral proteins which when injected trains the immune system to produce the required antibody response without causing the actual infection. Therefore, when the real virus strikes, the immune system is ready to handle the virus with ease. But how do we design a vaccine against a virus that mutates at such a high frequency? Yes, that is the real problem.
But wait. If you thought a mutating virus was all that we had to worry about, you are in for some real surprise. It so happens that there exists two types of HIV - HIV1 and HIV2. HIV1 is the more common type. This type contains 4 groups (M, N, O and P). Group M contains several strains, with nearly 30 percent variability between each strain. And the cherry on top of the cake? - People can get infected with multiple HIV strains at the same time! Can this get more complicated?!
“HIV is not really one virus, it is really like 50 million different viruses around the world right now.” - William Schief, Professor, Scripps Research
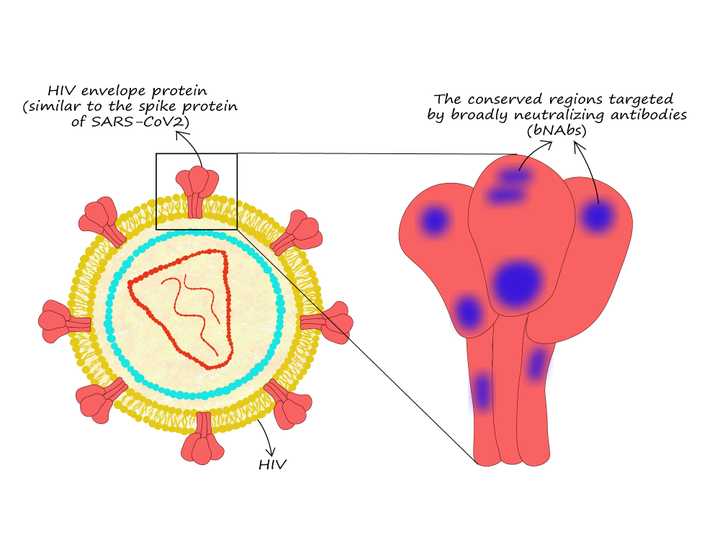
HIV envelope proteins and the regions targeted by the broadly neutralizing antibodies (bNAbs). © Sunaina Rao
Just like every cloud has its silver lining, this vexing hurdle in HIV vaccine development had its own silver lining moment in the early 1990s. Blood samples from a small fraction of HIV patients showed the first-ever evidence for the presence of antibodies that could recognise and inactivate or ‘neutralize’ a broad range of HIV strains. These antibodies were subsequently named broadly neutralizing antibodies or bNAbs. We now know that 20 percent of people infected with HIV produce bNAbs. The only setback is that they start producing these antibodies after the infection has significantly advanced.
Do you have the same question as I did when I first came across bNAbs? - How are these antibodies neutralizing a virus that keeps changing so rapidly? Intriguingly, studies (Burton and Hangartner, 2016) show that bNAbs target the Achilles’ heel of HIV. They neutralize specific regions on the viral envelope proteins that remain unchanged or are ‘conserved’ across different strains. These regions remain unaffected despite the mutations. However, it takes so much trial-and-error and fine-tuning for our bodies to generate such specialised antibodies that by the time they are actually made, the infection is beyond control.
So is this discovery a lost cause then? - Certainly not! The discovery of bNAbs was the first proof that antibodies indeed could be generated against such a deceptive virus. In the past decades, scientists have isolated over 200 different types of bNAbs and are now conducting trials (Mahomed et al., 2020) to see which of the bNAbs are best at preventing infection. Note that injecting bNAbs does not act as a vaccine since they are naturally degraded by the body and provide temporary immunity only. However, knowing which bNAb gives the best immunity, even if it is temporary, helps us design vaccines that could actively trigger our own B cells to continuously synthesise bNAbs and provide long-lasting immunity (Remember how vaccines work?).
So do we have a vaccine that can trigger bNAbs? - Not yet, but as we will see next, promising candidates are on their way.
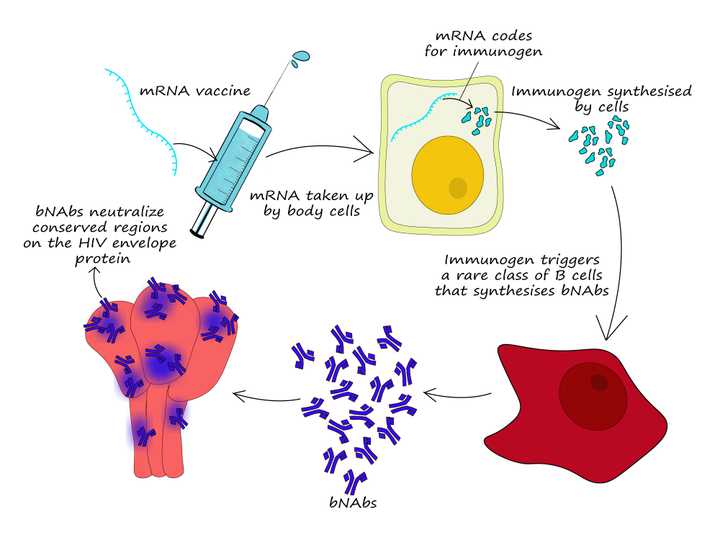
mRNA vaccine to trigger production of broadly neutralizing antibodies (bNAbs). © Sunaina Rao
The raving success of the COVID-19 mRNA vaccines from Moderna and Pfizer sparked an interesting question. Could we use an mRNA vaccine to trigger the production of bNAbs? In the early months of 2021, Moderna in collaboration with a few universities announced that they were to conduct a phase 1 clinical trial to test if an mRNA vaccine could trigger the production of a rare variety of B cells that could make bNAbs. This trial is based on the initial success of a clinical trial conducted by Scripps Research which showed how an artificially designed immunogen (a substance that induces an immune response) was able to successfully trigger this rare class of B cells.
Unlike the Scripps Research trial which introduced the immunogen itself, the mRNA vaccine candidate aims to inject mRNA which codes for this immunogen. The mRNA will be taken up by the cells, where the immunogen can be synthesized. This is expected to trigger the rare class of B cells that can make bNAbs. This clinical trial estimates the enrollment of 56 candidates and we should know how well it has fared by 2023.
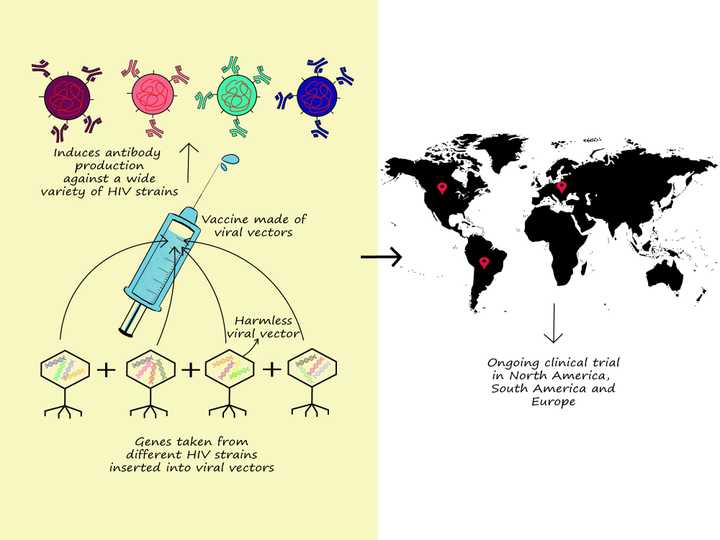
Right: Basic principle of the Mosaico trial. Right: Regions where this trial is currently being conducted. © Sunaina Rao
Besides bNAbs, there have been two other interesting approaches that have seen success in early clinical trials and have now progressed to phase 3 trials.
One of the studies, called the Mosaico study, involving over 3000 homosexual male participants from North America, South America and Europe, is investigating the use of virus vectors as vaccines in the delivery of a mixture or ‘mosaic’ of HIV genes. These genes code for specific HIV envelope proteins from a huge variety of strains from across the world.
So what is a virus vector you wonder? A virus vector is an artificially constructed virus lacking all of its genes required to cause an infection. It instead is designed to contain the genes of our interest (in this case the genes that code for the HIV envelope proteins). Viruses being excellent at specifically recognising and entering cells can hence be used as efficient delivery systems for the genes of our interest. Once the HIV gene mixture is delivered into the cell, the cell synthesises the viral proteins which in turn can generate an immune response against a wide variety of HIV strains. Whether this works effectively against HIV remains to be seen as the trial is estimated to be completed by 2024.
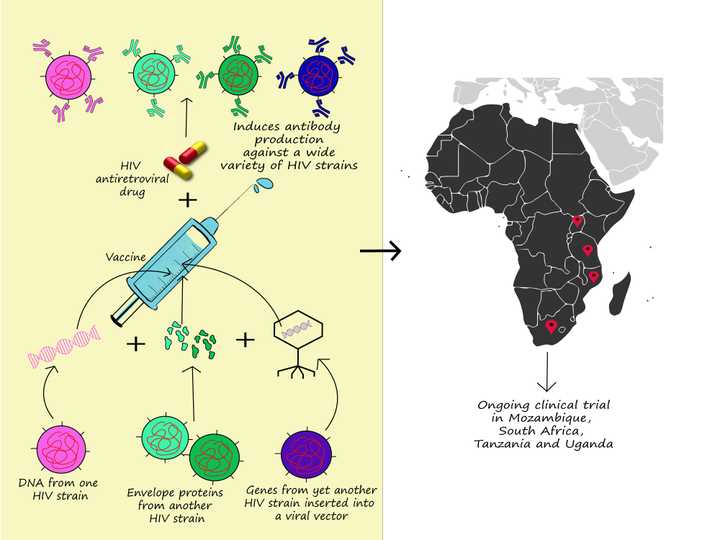
Right: Basic principle of the PrEPVacc trial. Right: Regions where this trial is currently being conducted. © Sunaina Rao
Another innovative study called the PrEPVacc trial, involving around 1600 candidates from Mozambique, South Africa, Tanzania and Uganda, utilizes a combination vaccine, meaning it combines a vaccine candidate with a non-vaccine drug. This combination too, in principle, aims to induce an immune response against a wide variety of HIV strains. However, it attempts to do so using an assortment of vaccine delivery methods including direct injection of viral proteins, DNA vaccine encoding viral proteins and viral vectors containing genes coding for HIV envelope proteins. Additionally, it combines this vaccine candidate with a drug commonly used in HIV antiretroviral therapy (TDF - Tenofovir disoproxil fumarate and TAF - Tenofovir alafenamide fumarate). Researchers conducting this trial believe this method is powered for a 70 percent efficacy. How far this may turn out to be true is for us to see when the trial is estimated to complete in 2023.
It is almost entertaining to be a spectator to this long-standing tug of war between the ever-changing viruses and the relentless scientists. It is even more fascinating how viruses, which lack even the most basic cellular structure and have still not found a place in our human definition of ‘life’, have posed such a big challenge, not just momentarily, but for decades on the run. So is 2023-2024 the victory year for the HIV vaccine? - That is for time to tell. Successful or not, perhaps a standing ovation for the persistence of scientific temperament is much deserved.