Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.
“When a plant goes to seed, the seeds are carried in all directions, but they only grow and live if they fall on congenial soil”- Stephen Paget here, way back in 1889 (Paget, 2003), wasn’t really talking about anything botanical, but was indeed suggesting that there might be a possibility that the disseminated cancer cells (seeds), can only colonise a specific site (soil), if and only if, it provides a congenial environment for it to grow.
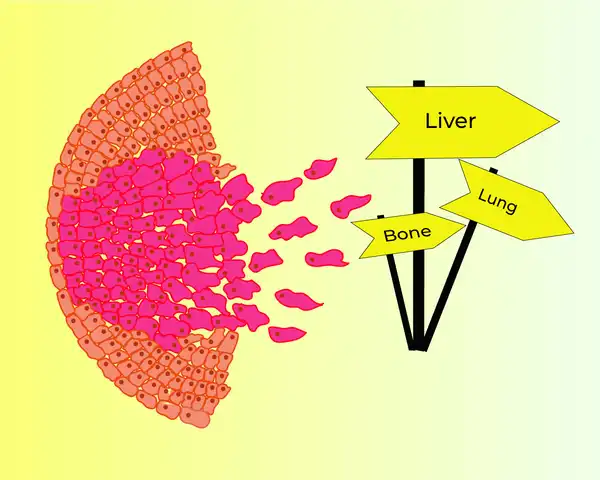
As you may already know, when a tumour begins to progress, the cells acquire the ability to spread, or metastasise, towards different sites. These cells enter the circulation, and are practically taken everywhere in the body, where they can now begin to grow and establish a successful metastasis. An interesting question we can ask here is - what decides where (or which organ) these circulating cancer cells will finally lodge? Is it entirely the property of the cancer cell, or as Stephen Paget suggested, can the destination site, called the metastatic site, by itself play a role in welcoming the cancer cells to successfully colonise in it?
One of the strong contenders of Stephen Paget’s theory was James Ewing, who claimed that metastatic spread was mainly a consequence of the dynamics of blood flow. It was only later in 1976 that two scientists, Fidler and Nicolson, showed (Fidler and Nicolson, 2017) the first ever evidence of organotropic metastasis, meaning cancer cells have preferential metastatic sites. They conducted a simple experiment using a metastatic melanoma (skin cancer) cell line. This cell line was administered into experimental mice via two routes – the intravenous route and the intracardiac route. They observed that independent of the route, cells always yielded lung colonies. Tumour cells indeed had organ preferences!
What we know now is that the original tumour itself plays a significant role in preparing its prospective metastatic site, also known as the pre-metastatic niche (Peinado et al., 2017). This can happen via certain tumour secreted factors.
A well studied example of a tumour secreted factor is the enzyme - Lysyl oxidase (LOX). LOX is an enzyme primarily responsible for the crosslinking of collagen fibres, which are very important components of tissues, and determine their stiffness. Interestingly LOX is secreted in excess by the rapidly growing tumor cells. The enzyme hence enters circulation and accumulates at different pre-metastatic niches.
A series of experiments conducted by Janine T. Erler and her group using breast cancer cell lines show how LOX regulates collagen crosslinking at the pre-metastatic niches (Cox et al., 2013). This crosslinking creates a fibrotic environment, thereby favouring the persistence and survival of the metastasising cancer cells in that region. This inturn leads to enhanced outgrowths and a successful metastasis. They further show that therapeutic targeting of LOX reduces tissue fibrosis, and thereby metastatic growth.
It would hence be interesting to wonder if we could have drugs in the future that could target not just the cancer cells, but also the pre-metastatic niche in a way that it becomes unfavourable for colonisation by the metastasising cells. As rightly suggested by Paget:
Although studying the properties of the seed is of importance, observing the properties of the soil may be of importance too.