Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.
The human body has been shown to have 37.2 trillion cells (Bianconi et al., 2013), all doing their own business in the respective tissues where they belong. But each of those 37.2 trillion cells originated from that very first cell, which came into being when the egg met the sperm! How then did that one cell multiply to make so many different types of cells? How does the cell on our toe behave so differently from the cell in our eye, even though they all have absolutely the same genetic material? Amongst the many factors that influence a cell’s differential behaviour, its immediate environment - called the cellular microenvironment, is a crucial one.
A cell is not a stand-alone entity. It is surrounded by its own microenvironment, which comprises a complex milieu of structural proteins, proteoglycans and enzymes (collectively called the extracellular matrix or ECM). It also contains a variety of cells such as the - fibroblasts, immune cells and endothelial cells. A cell’s microenvironment keeps it in place, and sends signals to the cell, allowing it to carry out its routine functions.
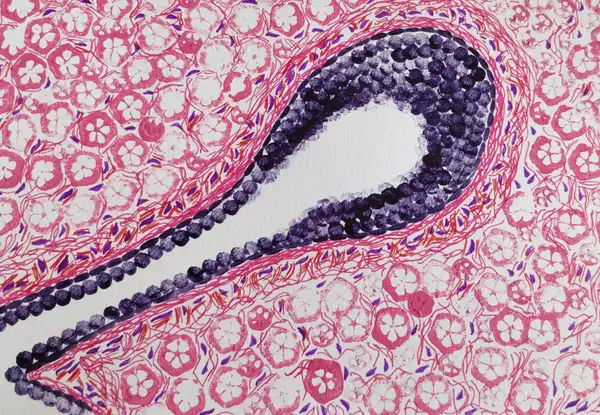
Mina Bissel and co-workers in the year 1989 conducted a very interesting experiment (Barcellos-Hoff et al., 2021) to demonstrate the importance of a cell’s microenvironment, using breast epithelial cells. Now before we discuss their experiment, let’s briefly understand the structure of the mammary gland . The mammary tissue at puberty, consists of a fat pad (a fatty structure) with several branching units called ducts and lobules. These ducts and lobules are like tubes, with a central opening. These units are lined by a class of cells called the epithelial cells. These epithelial cells have an interesting property. Their bottom surface is always firmly tethered to a fibrous, collagen rich entity of the ECM, and their upper surface always faces the central opening. This property of the epithelial cells is called polarity and it underlines its three dimensional architecture in the mammary gland.
Now during pregnancy an interesting transformation occurs in the mammary gland. Under the influence of hormones such as progesterone and prolactin, the ducts and lobules become more and more branched. The epithelial cells that line these units eventually secrete milk into the opening. This milk travels through the ducts and is finally released through the nipple, acting as the chief source of food for the newborn offspring.
In order to study these mammary epithelial cells, scientists routinely grow them (extracted from patient biopsies or as cultured cell lines) on plastic petri dishes. Considering that these dishes are 2D, unlike the tissues which are 3D, these extracted cells multiply and spread on a 2D plane. However, they show no glandular architecture, no polarity, and in no way represent the actual 3D conformation they exist in, in the body. And of course, they don’t secrete milk.
What Mina Bissel and co-workers did is that they grew these mammary epithelial cells on petri dishes coated with artificial ECM, comprising proteins such as collagen and laminin. What they observed as a result was incredible! They found that the same cells were now generating 3D, hollow spherical structures that somewhat resembled the ducts and lobules. Interestingly the cells also showed polarity, i.e. their bottom surface was anchored to the artificial ECM, and their upper surface was facing a central opening. Not just that, using immuno-precipitation experiments they showed that, under the influence of specific hormones, more than 80% of caseins, a type of milk protein, were secreted by the cells into the opening. The cells were making milk…once again!
What we can conclude from this experiment is the importance of the cellular microenvironment, more significantly the protein rich ECM in the functioning of the cell. Cells that had little idea of what they had to do when grown on a plastic dish, regained much of their functionality when grown on a protein rich ECM.
The role of the ECM can be fully appreciated in the context of tissue homeostasis. Our cells are constantly exposed to various kinds of stresses, and that would mean that at any given moment, our body may easily have numerous tumourigenic cells. But these cells are constantly kept in check by their microenvironment, preventing them from establishing full blown tumours.The cellular microenvironment hence acts as a guardian (Bissell and Hines, 2011). But very often in cancerous tissues, it is observed that these very guardians can behave as partners in crime and facilitate the cancer cells to metastasise.
Researchers are now trying to understand the signalling mechanisms that facilitate this partnership between cells and their microenvironment. Understanding this will not only give us better insight into the pathogenesis of cancer but will also help us understand in general, how tissue architecture and homeostasis are elegantly maintained in the body.