Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.

If we were to personify the DNA molecules in our body, these molecules would most certainly be deemed the VIPs of our cells. The DNA indeed receives presidential treatment and protection, with several types of machinery maintaining its integrity. This is with good reason of course - Our DNA harbours the crucial genetic information required for the functioning of the cell and helps pass this information to the next generation.
Among the many molecules that protect the DNA, the protection provided by an enzyme called telomerase has been studied for almost 40 years now. Decades of research has helped uncover one critical function of this enzyme - It helps maintain the length of the very ends of the DNA strands. These ends are called the telomeres.
Telomeres have been famously associated with their crucial role in preventing DNA damage. Additionally, the reduction in telomere length is considered to be one of the critical signs of ageing. In fact, shorter telomere lengths have been used as biomarkers for ageing for a very long time.
So, if telomerase helps maintain the telomeres, and telomeres play such a significant role in ageing, we have a clear formula for retarding ageing, don’t we? Just increase the levels of telomerase in our cells and we will be on our way to ever-youthful lives.
Unfortunately, the biology of ageing is a lot more complicated than that. Let’s explore why!
Biological ageing has been defined as the irreversible deterioration of an organism’s physiological processes required for its survival with time. Note that this is different from chronological age, which refers to the number of years an organism has lived, although the two can go hand in hand.
What comes to your mind when you hear the word ageing? Greying hair? Wrinkled skin? Aching joints? These certainly are some of the physiological manifestations of ageing. However, ageing, in reality, begins at the cellular level.
During the course of life, our cells face constant stress. The stressors can be both internal and external.
These stressors damage the cellular components, specifically the genetic material (DNA). This damage accumulates with time, leading to the gradual deterioration of the functionality of the cell. This is cellular ageing. These damaged, ageing cells eventually reach a point called senescence, wherein cells can no longer divide and are killed by the immune cells. So, aged cells ultimately retard the functioning of the organ and the organism as a whole.
Now, a key region of the DNA that gets damaged due to stressors is the telomere.
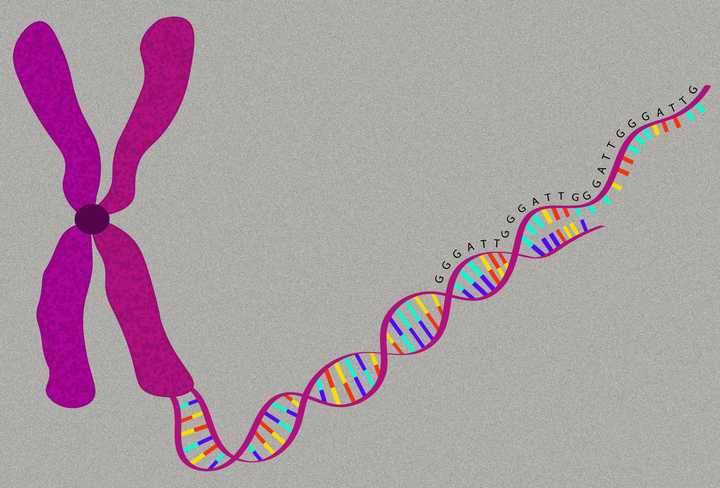
The ends of our DNA strands, called telomeres, have a distinct sequence of nucleotides - TTAGGG, that repeats itself 100s to 1000s of times. © Sunaina Rao
Telomeres are the specialised ends of the DNA double helix and first came into the limelight in the 1930s. Barbara McClintock, while working on maize, noted that the DNA strands without their ends were extremely unstable and prone to breaking.
We now know that these telomeres are not just mere ends. They have a distinct structure with a distinct sequence of nucleotides in the DNA - TTAGGG, that repeats itself 100s to 1000s of times. Additionally, a specific protein complex binds to these repetitive sequences, forming a ‘cap’ at the ends of the DNA double helix. This does a very important job - It protects the ends of the DNA strands from fusing. Fused DNA strands can lead to catastrophic DNA damage when the cell divides. Therefore telomeres help maintain the general integrity of the DNA.
However, telomeres do not remain entirely intact throughout the lifetime of an individual. Each time a cell divides, due to a technical issue called the end replication problem, a small portion of the telomere is lost. Hence the telomere gets shorter and shorter with each round of cell division. Once a critical length is reached, the cell becomes senescent and eventually dies. In fact, telomere lengths significantly reduce with age, from 11-15 kb (kb, kilobases = 1000 bases = 1000 nucleotides) long at birth, to about only 4 kb in the elderly.
So, if telomeres are of such paramount importance, our cells must have evolved some way to protect them, right? That is where the enzyme telomerase comes into the picture.

Elizabeth Blackburn and Carolyn Widney Greider won the Nobel Prize for the discovery of telomerase. Credit - Left: Science History Institute; Right: Keith Weller
The enzyme telomerase was first described in 1985 by Elizabeth Blackburn and Carolyn Widney Greider while they were studying the telomeres of a single-celled organism called Tetrahymena. The duo went on to win the Nobel Prize for their discovery.
What was so special about this enzyme? They observed that this enzyme had the unique ability to maintain the length of those invaluable telomeres. It helped to compensate for telomere loss due to the end replication problem.
Incidentally, our body cells also appeared to contain the very same enzyme.
So essentially, the telomerase, at least in part, should be able to slow down the overall ageing of our body. This is partly true, but it is not so straightforward.
Although the gene for telomerase is present in every cell of our body, the expression of those genes is almost negligible in most of the cells. The only cells in our body with high telomerase activity levels are our germ cells or the sex cells. This ensures that a newborn offspring has sufficient telomere lengths. As a matter of fact, telomerase activity is reasonably high in the embryonic stem cells (ESCs), however, the activity declines within 20 weeks of gestation. The adult stem cells in our body, which routinely replace dead and worn-out cells, also show detectable levels of telomerase activity. However, even this can never completely counteract the shortening of telomeres.
As unfortunate as this is, it poses interesting questions. Could we then artificially heighten telomerase activity in our cells? Could we consume foods with telomerase?
Sadly, no. Even if we did find foods with high telomerase levels, the enzymes in our digestive tract would almost completely denature this protein before it can be absorbed by our cells.
An alternative to this could be that we genetically engineer our cells to express more telomerase. This might help maintain long telomeres throughout the lifetime of an individual, which could help retard the ageing process. However, this can pose yet another, exponentially more serious problem - It might make the cells cancerous.
Cancer cells have the ability to divide uncontrollably, and one of the many things they do to achieve this is heightening their telomerase expression. Approximately 90 percent of cancers show enhanced telomerase activity. So essentially, tweaking telomerase activity can act as a double-edged sword - Too little can enhance ageing and too much might trigger cancer.
So, can altering telomerase activity in our cells help slow down ageing? This continues to be a long-standing debate in the science community. Perhaps our cells had an evolutionary advantage in maintaining low levels of telomerase? Until scientists solve this paradox, we can certainly follow the good old principles of healthy nutrition and exercise to keep our telomeres long and strong. Indeed consumption of foods like legumes, fruits, and nuts has shown a positive influence on those long beautiful tips of our DNA.
For further discussion, we welcome you to leave your ideas in the discussion section below.