Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.
Oxygen is one of the most vital molecules required for existence. We know the full story - first oxygen from the air is taken in by the lungs; then it is carried by haemoglobin present in the red blood cells to the different organs; here, the oxygen diffuses into the cells of the organs, directly partaking in the enzymatic reactions required to derive energy. But how do these cells actually sense the presence of oxygen? Our body has five main sense organs that help us sense things around us. But what about our cells? Do they have their own ‘sense organs’ that can help them detect oxygen? This was the exact question pursued by the 2019 Nobel laureates - Gregg L. Semenza, Sir Peter J. Ratcliffe and Willian G. Kaelin, Jr.
An understanding of the cell’s oxygen sensing abilities first began when scientists made a correlation between low oxygen levels in the blood, something called hypoxia, and the activation of the erythropoietin gene in rodent kidney and liver cells (Wang et al., 2006). They saw that hypoxia stimulated the production of a protein called erythropoietin (EPO) in these cells, which inturn stimulated the stem cells to make more RBCs. This thereby increased the oxygen carrying ability of the blood. So these kidney and liver cells were clearly showing the ability to sense the levels of oxygen in them, but what was the molecular mechanism behind this?
In the effort to understand how the erythropoietin gene is regulated, Gregg Semenza in 1991 hypothesised that the gene must be activated by specific factors. He successfully identified using mice models as well as human liver cell lines, that a specific region in the EPO gene, bound to at least two factors that were triggered by hypoxia. They called this factor, the Hypoxia Inducible Factor (HIF). (Wang et al., 2006)
On exploring further, Gregg Semenza and group identified that the RNA and protein levels of the two HIF1 subunits – HIF1α and HIF1β increased during hypoxia (1% O2), and rapidly decayed when the oxygen levels were restored back to normal (20% O2).
So essentially oxygen seemed to be degrading HIF 1.
With these experiments we were getting closer to deciphering the cell’s oxygen sensing abilities, but it wasn’t clear how oxygen regulated the degradation of HIF1.
At around the same time, Peter J. Ratcliffe and group were working on a syndrome called the Von Hippel-Lindau syndrome which is characterised by the formation of highly aggressive tumours. These tumour cells lacked a certain protein called the Von Hippel-Lindau tumour suppressor protein (pVHL).
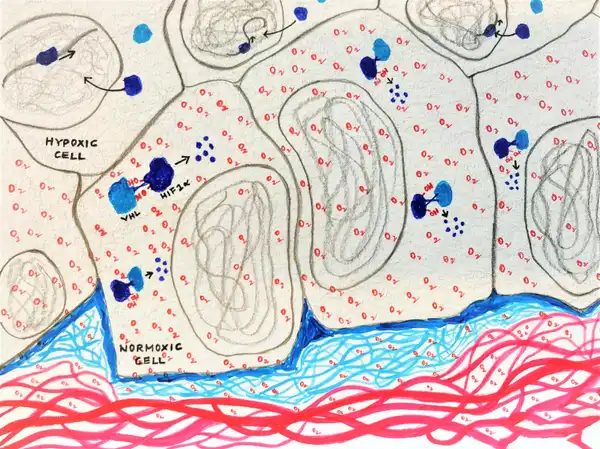
They hence concluded that the degradation of HIF1 α was dependent on its interaction with pVHL, which in turn was dependent on the presence of oxygen and iron.
But the question remained as to how oxygen and iron enabled the interaction between HIF1 α and pVHL, and how this interaction enabled the degradation of HIF1 α.
All the pieces of the puzzle finally came together when it was identified by both – the William G. Kaelin and the Peter Ratcliffe groups, almost simultaneously, that it was the hydroxylation of a specific amino acid residue in the HIF1 α protein that facilitated interaction between HIF1 α and pVHL. Peter Ratcliffe and team identified an enzyme responsible for this hydroxylation, which required oxygen and iron for its functioning. They termed it HIF 1 α prolyl hydroxylase (HIF-PH) (Ivan et al., 2007, Jaakkola et al., 2007).
So, the hydroxylation of HIF1 α allows pVHL to interact with it, which consequently targets it towards degradation.
These discoveries helped put all the pieces together, but in what way is it of significance to modern science and medicine? Firstly, it’s helped us better understand basic physiological processes including metabolism, immune response and embryonic development. Secondly, it’s helped us take huge leaps towards progressing certain therapeutic interventions such as in wound healing, anaemia and cancer treatment. Indeed, their efforts deserve much credit!