Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.

Diabetes is a serious metabolic disease that impacts millions of lives worldwide. In the year 2019, it was estimated that more than 400 million people across the globe were affected by diabetes. This number is sharply rising and is estimated to touch 700 million by the year 2045. This is approximately 11 percent of the world population. Of this, type 2 diabetes accounts for almost 90 percent of the cases.
As discouraging as these statistics may be, diabetes is not new to human society. Ancient Egyptian, Indian, Chinese and Arabic scriptures have tried to describe several signs and symptoms of diabetes, implying the disease was not unheard of in our ancestors. Not just in humans, interestingly the occurrence of diabetes is also found to be quite common in cats, dogs and a few wild animals.
So considering the vast prevalence of diabetes, how close is modern science to finding its cure, specifically type 2 diabetes? Let’s explore this, shall we?
Diabetes is defined as the state in which carbohydrate and lipid (fat) metabolism are improperly regulated by insulin.
Carbohydrates and lipids are the main energy-giving biomolecules in our bodies, and the efficient management of their levels is of utmost importance for the proper functioning of our cells. Now amongst the several enzymes and signalling molecules involved in the metabolism of these biomolecules, insulin is the most important one. Insulin is a hormone secreted by a specific set of cells in the pancreas, called the beta cells. This hormone is primarily responsible for the uptake of consumed glucose (a type of carbohydrate) by cells from the blood.
Diabetes is commonly classified into two categories:
Due to this improper functioning of beta cells and inefficient regulation of insulin, the uptake of glucose is hindered. This leads to the lack of glucose availability in the cells, in turn leading to elevated levels of glucose in the blood.
In the past 20 years, research in the field of type 2 diabetes has resulted in a plethora of drugs that target specific pathways of glucose metabolism. These certainly have helped millions of diabetes patients keep their blood sugar levels in control. However, this also means a lifelong dependency on medication.
Alternatively, a recent study published in the journal The Lancet shows how an intensive lifestyle intervention resulting in significant weight loss led to diabetes remission in 60 percent of the participants. Diabetes remission essentially refers to the maintenance of healthy levels of blood sugar without needing any medication.
Indeed this is a very encouraging study. Unfortunately, there is little evidence that remission is permanent.
Many other scientists have inclined towards more permanent solutions such as pancreatic transplantation and beta-cell transplantation in the treatment of both type 1 and type 2 diabetes. Nevertheless, these options come with their own share of problems. Transplanting cells from other individuals most often leads to graft rejection and severe immune complications.
Fortunately, scientific research does not halt due to adversities. In fact, it is a gradual process where each step is a fine refinement of the previous. In this article, we will dive into three interesting recent scientific advancements that aim to provide a more permanent solution to type 2 diabetes.
All of these strategies primarily focus on increasing the invaluable insulin-secreting beta-cell population of the pancreas.
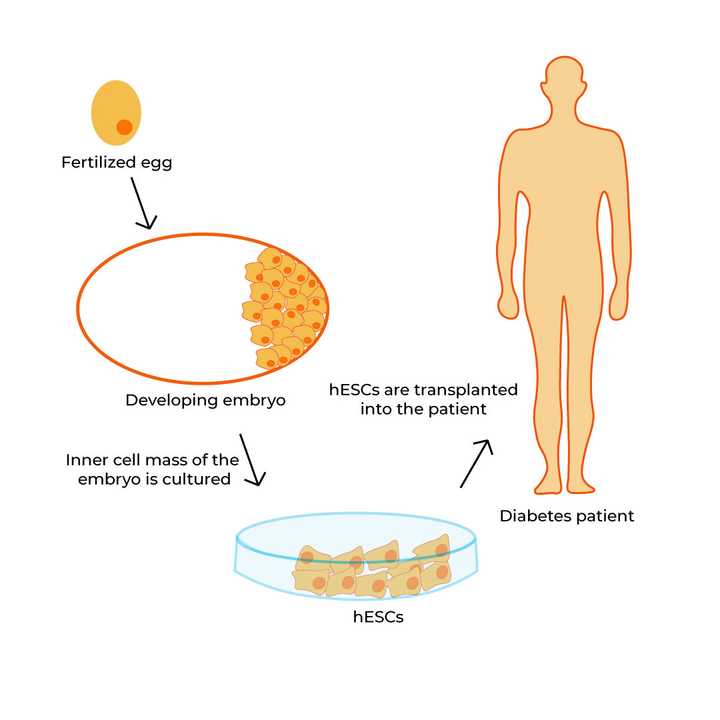
hESCs (derived from a fertilized egg) are cultured under laboratory conditions and transplanted into diabetic patients. © Sunaina Rao
A majority of cells in our body are highly specialised and are bound to specifically defined roles. However, there exists a small population of cells in our body that are relatively unspecialised and have the remarkable potential to develop into multiple cell types. These are called stem cells and they play a significant role in replacing the dead and worn-out cells of the body.
Stem cell replacement therapy for diabetes taps into this exact potential of stem cells.
Now, several completed and ongoing clinical trials in diabetes treatment have employed a specific variety of stem cells called the human embryonic stem cell (hESC) which can be derived from fertilised human eggs. These hESCs are first encapsulated in a biocompatible material and then transplanted into patients, where they mature into fully functional, insulin-producing cells.
hESCs have been reported to show lesser immune rejection, making them a promising strategy for treating diabetes. Scientists are now in the process of rigorously testing this kind of therapy in the hopes of achieving long term safety and efficiency in maintaining normal blood glucose levels.
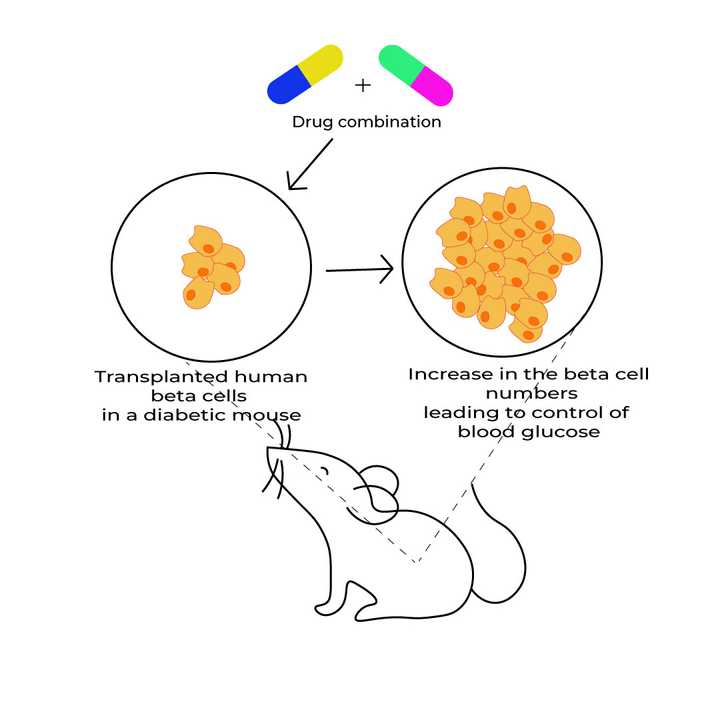
The proliferation of transplanted human beta cells in a diabetic mouse is brought about by treatment with two specific drugs - 1. Inhibitor of the dual-specificity tyrosine-regulated kinase 1A (DYRK1A); 2. Activator of GLP1R (Glucagon-like peptide-1 receptor). © Sunaina Rao
In a study published in the journal Science Translational Medicine, scientists aim to provide a lasting solution to diabetes without the need for any kind of transplantation and the hassles that come with it.
They show that under laboratory conditions when human pancreatic beta cells were treated with a combination of two molecules, it could bring about a significant increase in the rate of proliferation of the cells, accompanied by the actual increase in the number of beta cells. One of the two molecules is an inhibitor of a specific enzyme called the dual-specificity tyrosine-regulated kinase 1A (DYRK1A), and the other belongs to a group of widely used diabetes drugs that activate a specific receptor called GLP1R (Glucagon-like peptide-1 receptor).
What is even more encouraging is that this drug combination was able to enhance human beta-cell proliferation, secretion of human insulin and control blood glucose levels in diabetic mice transplanted with human beta cells.
Considering that this method does not involve transplantation and uses a drug that is already widely used in the market, the authors of the study believe that this method is both cost-effective and scalable, having broad implications on diabetes worldwide. However, scientists are still in the process of determining the safety and long term sustainability of this technique. Moreover, we still need to see how this method fares in humans.
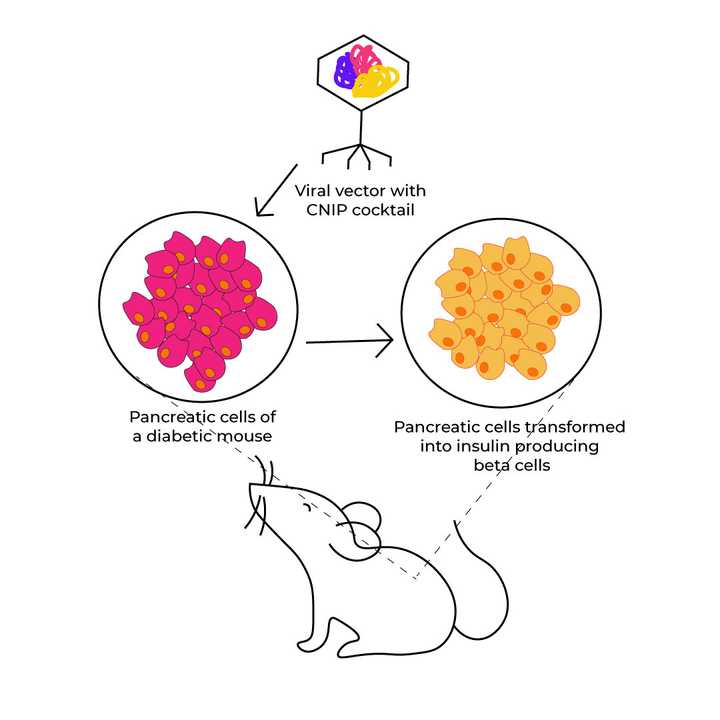
Pancreatic cells of a diabetic mouse are transformed into insulin-producing beta cells on treating with the CNIP cocktail. © Sunaina Rao
The last technique we will discuss here pertains to a study published in the journal Current Pharmaceutical Biotechnology and is comparable to the previous study. However, instead of expanding the already existing beta-cell population, the scientists aimed to transform a population of pancreatic cells into insulin-secreting beta cells.
To achieve this, the researchers used a cocktail of three biomolecules, which they called the CNIP (Cellular Networking, Integration and Processing) cocktail. This cocktail was injected into the pancreas of adult diabetic mice using a non-infectious viral backbone, called a viral vector, as the delivery vehicle.
Incredibly, they found that this cocktail was successfully able to induce the formation of insulin-producing beta cells in the pancreas. This in turn resulted in normalised fasting and postprandial blood glucose in the diabetic mice.
Considering the simplicity of application, scientists believe that this CNIP cocktail has the potential to be used as a preventative, treatment or even cure for diabetes. It is yet to be known however if the same results hold in humans.
Diabetes is without question a hot area of study and the research we talk about here is indeed just the tip of the iceberg! Although individual studies by themselves may not give us all the answers, it is ultimately the amalgamation of these studies, and many more, that could help us inch closer to the cure for this long-standing illness.