Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.
In the previous article – ‘How does the immune system work – Part1’, we see how when a virus enters our body, it is the physical barriers and the innate immune system that respond first. We see how the ‘Antigen Presenting Cells (APCs)’ engulf and digest the pathogens, presenting bits and pieces of the digested material called ‘antigens’ using the ‘class 2 MHC (Major Histocompatibility Complex)’ receptors on their cell membrane.
The APCs then travel to the nearby lymph nodes where they present these antigens to the cells of the adaptive immune system residing there. These then bring about the much-awaited targeted attack against the viruses and the virus infected cells.
So, what are these cells and how do they launch such a specific attack? For a specific attack, the virus particles replicating inside the infected cells, as well the newly made viruses that have come bursting out from the dying cells, need to be eradicated. While the former are found inside the cells, the latter would be found outside, in the tissue spaces and circulation. To eliminate these two separate sets of viruses, we need two separate sets of immune cells – The T cells and the B cells. Let me introduce you to them.
T cells are white blood cells (WBCs) produced from the stem cells residing in the bone marrow. However, they mature in the Thymus, hence the name T cells.
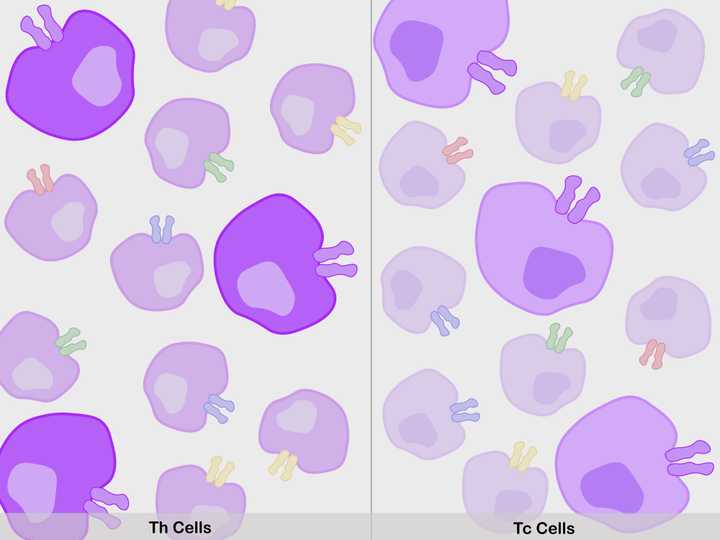
Millions of T cells are produced (Th cells – Dark purple, left; Tc cells – Light purple, right), with membrane TCRs of varied antigenic specificities (purple, red, blue, green, yellow etc.). One type of T cell population among the millions (ones with purple receptors), can recognise the viral antigens of the current infection. These get selected. © Sunaina Rao.
The newly formed ‘immature’ T cells undergo a stringent training process in the thymus. After all, fighting an infection requires a specific skill set and the human body does not take this lightly! So, what happens in this training?
First, the immature T cells begin to express an important receptor on their membrane, called the ‘T Cell Receptor (TCR)’. The TCR should be able to bind specifically to foreign antigens, presented to it by two types of cells:
Now, considering that the human body during its lifetime is exposed to a large spectrum of pathogens/antigens, it produces millions of T cells, all with TCRs of different specificity. One specific T cell population among the millions, will surely be able to identify and bind the viral antigen of the current infection.
Once the thymus ensures that all the T cells have a functional TCR, the cells are triggered to further evolve into two separate functional sets of cells – the ones with TCRs that recognise antigens presented by the APCs, called the T ‘Helper’ (Th) cells, and the ones with TCRs that recognise antigens presented by the infected cells, called the T ‘Cytotoxic’ (Tc) cells.
Finally, these two sub types of T cells are checked to see if their TCRs also happen to strongly bind and react to proteins made by our own body. If they do, then we have a recipe for disaster, as these immune cells will also begin to attack our own body cells (Autoimmunity). Such cells are hence eradicated.
These Th and Tc cells that pass this selection process, containing functional TCRs against varied foreign antigens, are now mature and are hence called the ‘mature’ T cells. They then move on to the lymph nodes, where they patiently await an infection.
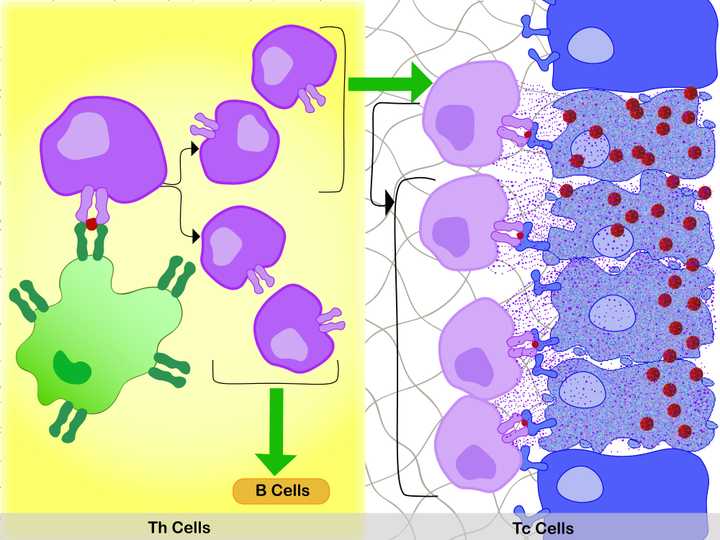
Left: In the lymph node, the specific interaction between the MHC 2 membrane receptor (dark green) of the APC (light green), the bound antigen (red), and the membrane TCR of the Th cell, activates the Th cell. Activation leads to production of two sets of daughter cells. One that activates (green arrow) the Tc cells and the other that activates the B cells. Right: In the tissue space, the specific interaction between the MHC 1 membrane receptor (dark blue) of the infected cell (light blue, shrivelled up cell), the bound antigen (red), and the membrane TCR of the Tc cell, activate the Tc cell. With additional stimulus from the Th cells (green arrow), the Tc cell divides. The daughter cells bind to the infected cells in a similar manner, releasing destructive enzymes (purple dots) into them. This specifically kills the infected cells and the viruses they harbour. © Sunaina Rao.
The mature T cells do not trigger an immune response until they are activated. They need to be given specific instructions by the cells of the innate immune system. So, let us take a look at how the body instructs the T cells into action during an active infection.
First, the APCs pick up antigens from the site of infection and bring it to nearby lymph nodes, where the Th cells are waiting. As previously mentioned, there is one type of Th cell population with membrane TCRs, that can specifically recognise and bind to the antigens presented by the APCs with their MHC 2 membrane receptor. This is like a brief handshake between the two types of cells. This highly specific interaction between the MHC 2 of the APC, the bound antigen, and the TCR of the Th cell, activates the Th cell.
Activation leads to rapid cell division and expansion of that type of T cell population. Hence, this T cell population gets ‘selected’. The daughter cells produced belong to two categories:
Hence these cells are aptly called the ‘Helper’ T cells, as they help stimulate other immune cells, hugely improving their functionality.
So, that is about the Th cells. Now, let us see how the Tc cells are activated.
During an infection, the Tc cells move towards the infected cells, and just like in the case of the Th cells, one type of Tc cell population gets selected due to its specificity for the antigen presented by, in this case the infected cells. This highly specific interaction between the MHC 1 membrane receptor of the infected cell, the bound antigen, and the membrane TCR of the Tc cell, activates the Tc cell. With an additional stimulus by the Th cells, these cells rapidly divide (remember how one set of daughter Th cells activate the Tc cells?).
The daughter Tc cells again bind to the infected cells in a similar manner, releasing destructive enzymes into them. This results in the targeted death of the infected cells and the viruses they harbour.
The body hence effectively eliminates infected cells and the viruses inside them. But what about those viruses outside the cells – the ones that have gotten into the tissue spaces and circulation? - We need antibodies for that.
I know this is a slightly complex topic to read and understand. If you were able to maintain your focus until now, kudos to your enthusiasm! Let’s move on.
The B cells, just like the T cells are made in the bone marrow. However, unlike the T cells, these mature in the bone marrow itself, hence the name B cells.
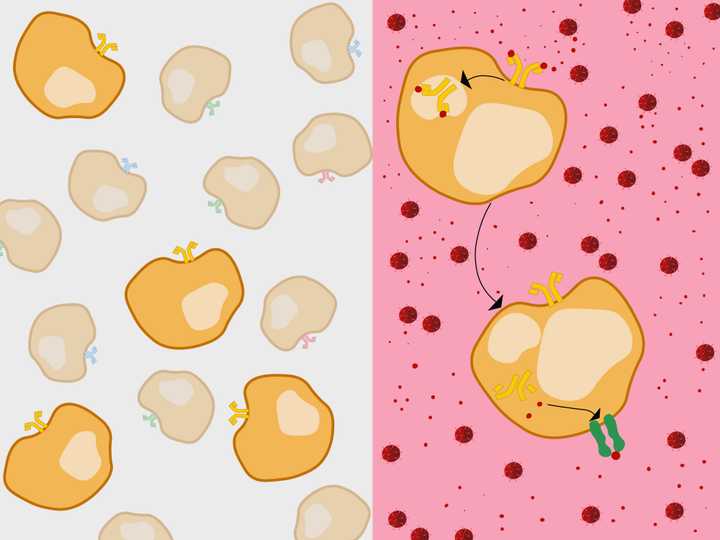
Left: Millions of B cells are produced (orange), with membrane BCRs (Y shaped molecules) of varied antigenic specificities (orange, red, blue, green, etc.). One type of B cell population among the millions (ones with orange receptors), can recognise the viral antigens of the current infection. These get selected and enter the circulation and lymph nodes. Right: In circulation, the B cell internalises the membrane BCR bound to the antigen (red). It then presents the antigen using its MHC 2 membrane receptor (dark green). © Sunaina Rao.
The newly made immature B cells undergo maturation in a manner similar to the T cells.
The cells first undergo expression of a specific receptor, here called the ‘B Cell Receptor (BCR)’ on their cell membrane, that can identify foreign antigens. Similar to T cells, millions of B cells are produced, with BCRs of different antigenic specificity. One type of B cell population among them should be able to identify and bind the viral antigen of the current infection.
Following this, cells with BCRs that react to proteins made by our own body, are eliminated to prevent autoimmunity. The mature B cells now enter circulation and the lymph nodes, awaiting an infection.
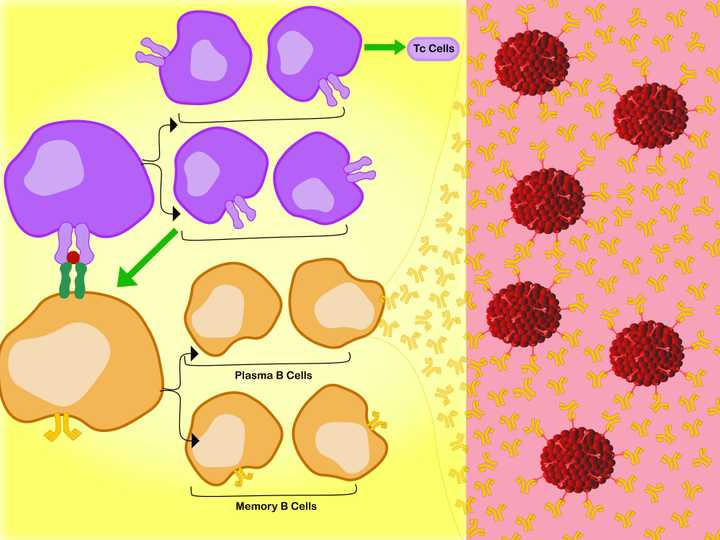
Left: In the lymph node, the specific interaction between the MHC 2 membrane receptor (dark green) of the B cell (orange), the bound antigen (red), and the membrane TCR of the Th cell, activates the Th cell. Activation leads to production of two sets of daughter cells. One that activates (green arrow) the Tc cells and the other that in turn activates the B cells. The activated B cells proliferate into Plasma B cells and Memory B cells. Right: The Plasma B cells make antibodies (orange Y shaped molecules), which enter the circulation and tissue spaces. They bind free floating viral debris, as well as regions of whole viruses (red), and neutralise their action. © Sunaina Rao.
During an active infection, the mature B cells which have BCRs specific for the current viral antigen, are able to bind to the antigens freely floating in the blood and tissue spaces and react to it. How they react to it mainly depends upon what kind of antigen it is. If it is a protein antigen, which is mostly the case during a viral infection, it needs a T cell to activate it. What needs to be remembered here is that these cells, unlike T cells, do not need antigens to be presented to them by the APCs. They can bind to freely floating antigens. So how do the T cells activate the B cells?
The antibodies secreted by the plasma B cells, are small Y shaped protein molecules, which are similar to the BCRs made by the parental B cells, in structure. The only difference is that these are not membrane bound but are actually released outside into circulation. Hence, they have the same antigenic specificity and can bind to the viral antigens of the current infection.
These molecules enter the circulation and tissue spaces, and bind floating antigens, which includes viral debris, as well as regions of whole viral particles. This neutralises the functioning of the virus and targets it for degradation by other components of the immune system (which we will not dwell into in this article, perhaps for the future). This effectively eradicates all infectious particles.
While most of the immune cells die shortly after the infection is over, the memory B cells remain behind. The memory B cells have membrane bound BCRs, which is again similar in structure to the parental BCR. In case of a reinfection by the same virus, the memory B cells can recognise antigens much faster, and can get activated by very low levels of the antigen. This creates an immediate immune response resulting in production of specific antibodies.
Even before you know it, the infection is taken care of!
So, what we see here is essentially an interplay between the innate and adaptive immunity. While the former helps the body produce an immediate, but non-specific response, the latter helps strategically eradicate the infected cells, the viruses inside the cells, and the viruses in circulation. However, the adaptive immunity takes its own sweet time to do so (almost 10-12 days to make the antibodies).
We need to note however, is that this story is indeed way more complicated than what has been described here. It involves several other cells and molecules, each playing its own important role, in a massive network of events, which we call an immune response.
Although this sends out the notion that our immune system has gotten it all figured out, there still exist several loopholes. For example - why are some infections fatal or near fatal? Yes, we have discovered several antibiotics and vaccines for such infections, but there still exist some infections – like HIV and Tuberculosis that we do not completely understand just as yet. Why is that? What makes these unicellular infectious agents outsmart us – The multicellular, and apparently ‘highly evolved’ beings?
Now that is a topic for another article!