Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.
You are preparing a big meal for dinner, so you are chopping your vegetables at high speed. Just as you begin to feel like you have everything under control, you hear your phone ring. You lose focus and the inevitable happens - you wedge your knife right into your finger with full force.
Ouch!
Your finger starts bleeding profusely, and the pain makes it hard for you to think. Even so, you rush to the sink and run some water on the wound. Fortunately, a few minutes later, the bleeding reduces. You cover the wound with a plaster and resume chopping your veggies, this time with a lot more care. Interestingly, when you return to your wound a few days later, your skin looks as good as new!
This process of wound healing might seem like a little magic trick performed by our body. But indeed, there is a lot of happening behind the scenes. There exists a whole crew of cells and molecules which dedicate their lives to simply healing our wounds, and there is good reason for this - Wounds are perfect entry points into our bodies for infectious agents like bacteria. Hence, our body takes great care in healing our wounds and it does so in 4 phases (Velnar et al., 2013, Rodrigues et al., 2018).
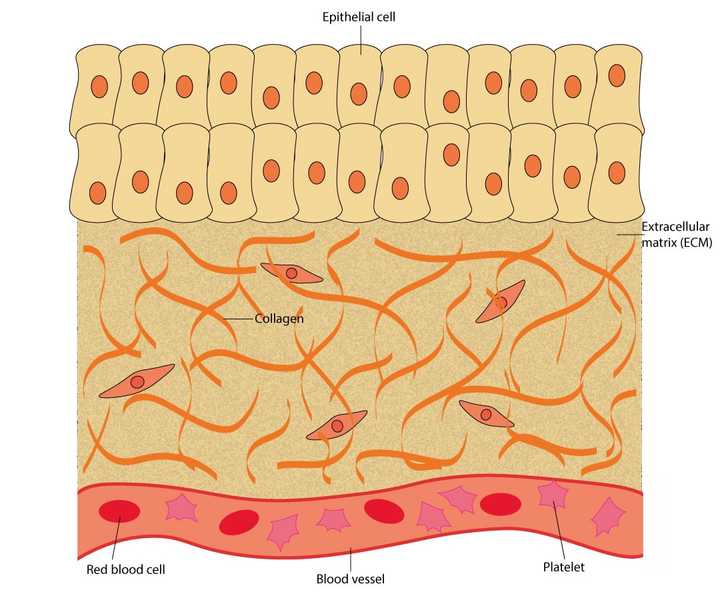
The uninjured skin is made up of an outer layer of epithelial cells. Beneath this outer layer lies the extracellular matrix (ECM), made up of proteins like collagen. The tissue also carries several blood vessels which contain blood cells like the red blood cells and platelets. © Sunaina Rao
When we cut our skin, it essentially damages the outer layer of cells (called the epithelial cells), the proteins packed beneath this outer layer (called the extracellular matrix or ECM) and the blood vessels that run across the tissue.
The ruptured blood vessels ooze out blood and can cause us to lose essential nutrients. Hence, it is important that the bleeding is stopped immediately. To achieve this, the blood vessels in the damaged area rapidly become narrower. This temporarily reduces bleeding.
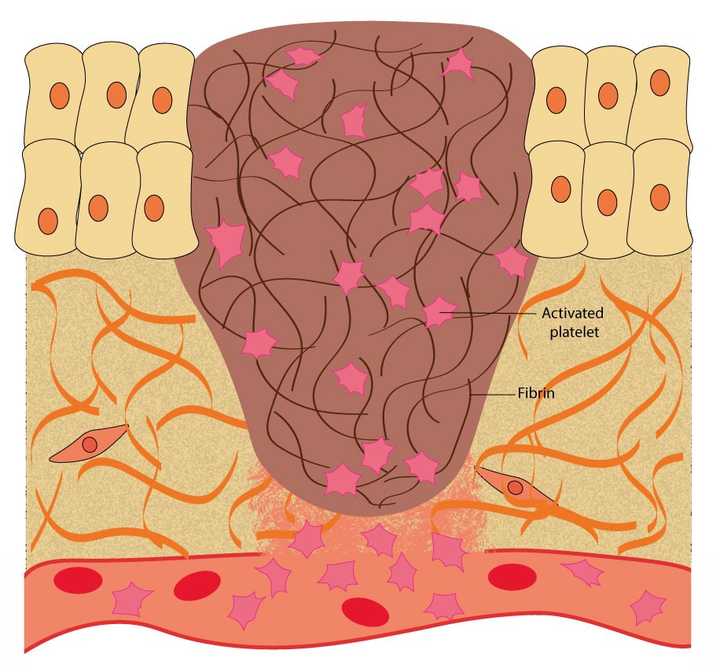
In the haemostasis phase, the blood oozing out of the blood vessels is blocked. This is achieved by the platelets, which help in the synthesis of a protein mesh made of fibrin. © Sunaina Rao
The blood that comes oozing out contains a variety of blood cells. Amongst these are small cells, called the platelets, which play a vital role in wound repair. When the platelets come in contact with specific proteins present in the ECM, like collagen, they get activated. Activated platelets release small proteins called clotting factors. These factors help create an insoluble mesh in the wound area, mainly made up of a protein called fibrin. The protein mesh blocks the blood from oozing out.
This is the reason why we might observe that within a few minutes of an injury, the bleeding considerably stops (if we are healthy). This is fibrin in action.
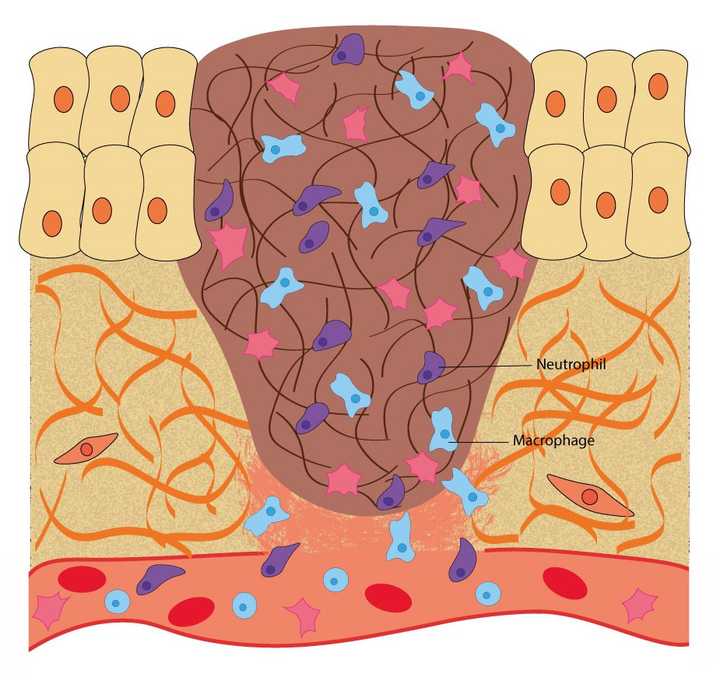
In the inflammatory phase, macrophages and neutrophils enter the wound area and kill any infectious agents like bacteria and fungi. © Sunaina Rao
Once the important job of stopping the bleeding is accomplished, the next important step begins - Clearing the wound area of any kind of infectious agents, like bacteria or fungi.
In the haemostasis phase, the platelets begin to release certain chemicals called chemoattractants. Attracted by these chemoattractants, the immune cells in the blood - like the neutrophils and macrophages, exit the blood stream and enter the wound area. They use the fibrin network for support to navigate through the wound area. On encountering any infectious agent, they eat up or ‘phagocytose’ the agent, thereby eliminating the possibility of an infection. Once their job is done, they kill themselves by a process called apoptosis.
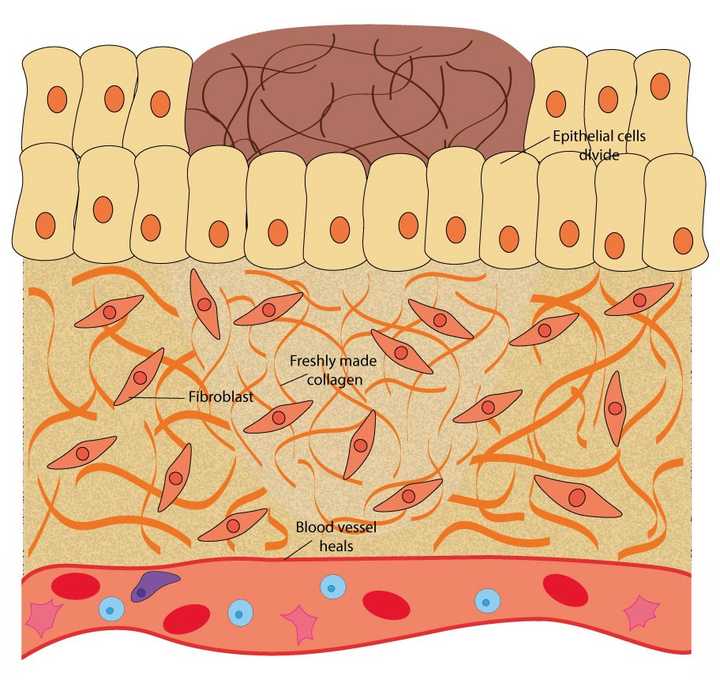
In the proliferative phase, the epithelial cells divide to cover the wound opening, the fibroblasts in the extracellular matrix (ECM) secrete fresh collagen and the ruptured blood vessels heal. © Sunaina Rao
From the 3rd day to around 2 weeks post injury, the actual repair of the injured tissue takes place.
If you remember, we previously mentioned a protein rich region, called the ECM, that lies beneath the outer layer of cells. This ECM also contains specific cells called fibroblasts that markedly increase in number in the proliferative phase. These cells secrete a thick fibrous protein called collagen into the wound area, which replaces the initially formed fibrin mesh. Collagen is one of the most important proteins present in skin and provides strength to the wounded area.
It is important to note that although the blood is stopped from oozing out, the blood vessels still remain ruptured. Therefore, the ruptured blood vessels need to be fixed too. This is achieved by certain growth factors released by the platelets in the haemostasis phase. These growth factors help the cells that line the ruptured blood vessels to divide, thereby forming fresh blood vessels in the wound area. This process is called angiogenesis.
Additionally, the outer layer of epithelial cells also begin to divide around the edges of the wound and cover the opening of the wound.
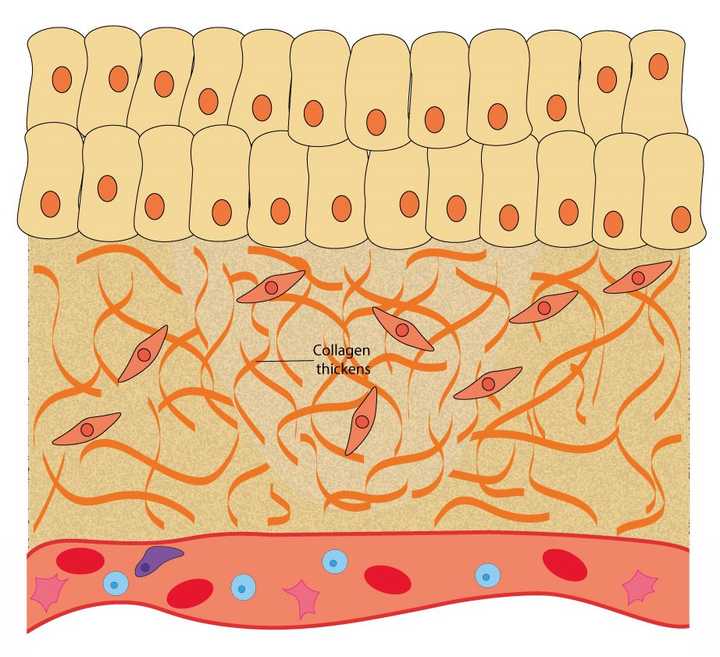
In the remodelling phase, the secreted collagen thickens, providing more strength to the tissue. © Sunaina Rao
Once the tissue is repaired, most of the hard work in rescuing the tissue is done. However, the wound area continues to be remodelled for up to 1-2 years post injury. This Is the body’s attempt to return the tissue to a state as close as possible to the uninjured one.
In this phase, the collagen fibres secreted in the previous phase thicken, providing the area with more strength. Gradually, all the cells that were recruited to the wound for its repair, like the immune cells, fibroblasts etc., reduce in number. What is left behind is a fully functional scar tissue.
Although in most of us, wound healing may be quick and efficient, there are many who suffer from wounds that don’t heal for several weeks. These are called chronic wounds and can be observed in people who are aged or diabetic. New age gene and protein technologies are helping us better understand wound repair. Further, scientists are now using 3D skin models to study wound healing in labs. These attempts can possibly take us closer to designing better remedies for patients suffering from chronic wounds (Wilkinson and Hardman, 2020).