Everything you need to know about stem cells
What are stem cells, and what makes them so unique? What are the different types of stem cells, and how have they impacted modern medical science? Here is us answering all your questions about stem cells.
Delivering chemotherapeutic drugs to cancer cells is a booming industry by itself. Why? Because cancer cells are similar to normal cells in a lot of ways, making it hard for drugs to actually differentiate between the two. By the time the drug has some effect on the cancer cells, there are already a few notorious cells that get away, evolve, and become resistant to the drug. Additionally, a large portion of the drug molecules are lost in transit as they move through the bloodstream, and may not even make it to the target cells. So what becomes clear here is that this is a tricky disease to tackle, and requires some strategic drug delivery. A strategy that not only brings efficiency but also allows high specificity.
Research now shows that nanoparticles can be one such strategy. (Dadwal et al., 2018, Goenka et al., 2013)
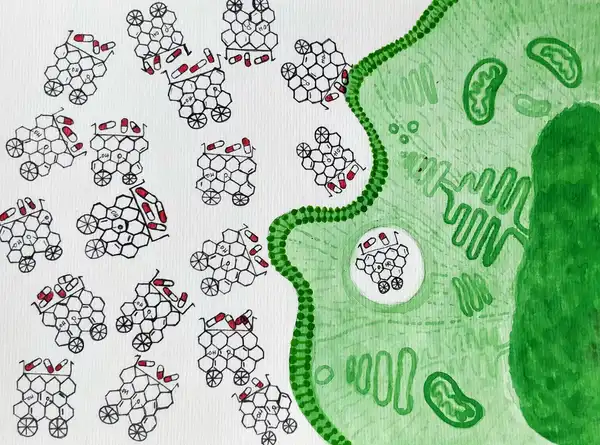
A nanoparticle as the name rightly suggests is a particle that has its size in nanometres (1-100 nm), which is about 2000 times smaller than the diameter of the human hair. These particles can be in the form of chemical polymers, small lipid molecules, viral particles and so on, onto which chemotherapeutic drugs can be loaded, followed by injection into the bloodstream. So, what we essentially want are molecules that are so minuscule in size, that they can easily make their way through the bloodstream, but also have enough surface area to hold on to a good number of drug molecules. Let’s for convenience compare this to a truck with good cargo space.
What we need to remember is that a nanoparticle is not the sole traveller in the bloodstream. It travels with millions of other cells like the RBCs, WBCs, and other biomolecules, all heading in their respective directions. Hence the nanoparticle must be relatively inert to its environment to be able to effectively deliver the drug without causing any toxicity.
Once the nanoparticles have reached the cancer cells, they should be able to enter the cell and release the drug into the respective compartment in the cell. Additionally, we also want the particles to deliver the drug specifically to the cancer cells while keeping their interaction with normal cells minimal. To enable this, nanoparticles are often equipped with molecules which specifically bind to the receptors present on the surface of cancer cells, increasing their interaction with cancer cells as opposed to the normal cells. Once inside the cell, due to the change in the pH, the drug loses its affinity to the nanoparticle and is thus released.
Indeed nanoparticles behave very much like heavy duty trucks with a GPS tracker. Not only do they hold on to a good load of drug molecules, but they also know exactly where to take them!