
5 unique autumn things and the science behind them
Autumn brings with it many changes in the weather as well as in plant and animal behaviour. But why these changes and what is the science behind them?

Perhaps it is not an overstatement to say that plastics have taken over our lives in the past few decades. Beginning with the huge surge in production during the 1950s, we have made more than 8.3 billion tons of plastic ever since. If you take a quick glance around you, most of the items you find might have some component of plastic in it. But what makes plastic so special?
The answer lies in its chemistry. Chemically, plastics are polymeric substances. A polymer is a molecule made of repeating monomeric units, linked by strong bonds. The polymers that make up different kinds of plastics can have thousands of such repeating units. This gives them a large molecular size and a specific physical form. Owing to this chemical property they can be shaped and moulded into a variety of products, from the thin plastics used in packaging of food, to the more hardy ones used in toys and electronic gadgets. Considering its versatility, perhaps plastics can be rightly called a man’s best friend.
So what is the problem? Ironically, the problem lies in the same chemistry, and the horrendous way in which we have managed it.
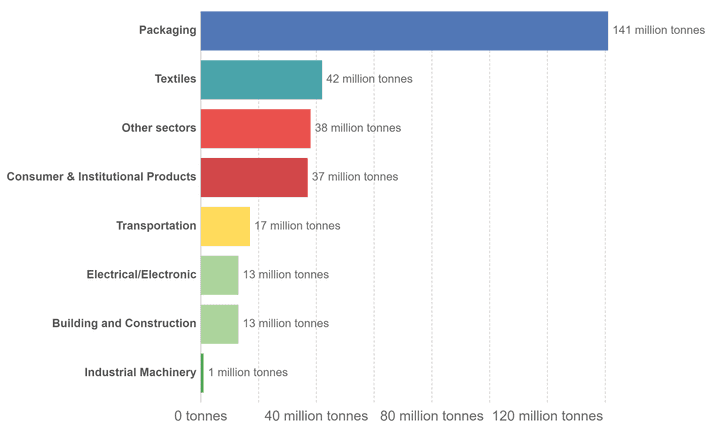
Plastic waste generation by different industrial sectors in the year 2015. Credit: Our World in Data; Source: Geyer et al., 2017.
Plastics and us humans have indeed been struggling with some sort of a love-hate relationship. Interestingly however, polymers are not so new on this planet. All living organisms have components made of polymers. Many of these are quite resilient to enzymatic action like the cellulose in plants and the chitin in animals. But organisms have had millions of years to evolve the right enzymes that can bind to, and break down these polymers into their respective monomers. Hence establishing a continuous turnover of raw material.
Most of the plastics on the other hand are synthetic polymers, made artificially by man in the past few decades. Nature has not had enough time to evolve the right enzymes to break these down. This means it could take hundreds to thousands of years for plastics to be decomposed back to their monomers.
So how much plastic have we accumulated on our planet? An estimate shows that out of the all plastic manufactured between 1950 and 2015, almost 55% landed up as waste in landfills and only 6-7% was recycled. A staggering 8 million tons of plastic ended up in the ocean in the year 2010 alone. These highly mismanaged, non-degradable plastics in the biosphere, undoubtedly pose a serious threat to the thousands of species that exist in it. Additionally, the monomeric units that make up plastics are normally obtained from natural resources such as oil, natural gas and coal. Hence, not being able to degrade plastic, disrupts the continuous turnover of raw material and the much needed circular economy.
Needless to say we have an environmental crisis at hand and something urgent needs to be done about it.
This problem can be approached in many ways. To begin with the production point of view, we can try to replace traditional plastics with the more degradable plastic options that are now stepping into the market. Next, from the consumer point of view, we can reduce consumption of plastic and aim to improve its management systems, both at the grass root as well as the national/international level. This means lesser plastic waste landing up in the ocean and as landfills, and more being recycled.
But what do we do about the millions of tons of plastic that has already been dumped into nature. We need to find innovative ways to degrade those. One such emerging way is to employ microorganisms that can do the work for us - something called bio-degradation.
Emerging data in the recent few years has shown that a variety of organisms, have evolved ways of degrading plastic into its constituent units. One of the most promising organisms is the bacteria Ideonella sakaiensis, isolated from outside a plastic bottle recycling facility. Researchers have shown that this new species of bacteria, uses polyethylene terephthalate (PET), a common polymer used to make beverage bottles, fibers for clothing etc. as their major source of energy. With the help of two enzymes - PETase and METase they metabolise PET into their subsequent monomers - terephthalic acid (TPA) and ethylene glycol (EG).
It is indeed remarkable how this bacteria has evolved such an efficient way to degrade something man-made. The immediate next question however, is if there is a way to use this bacteria for our advantage?
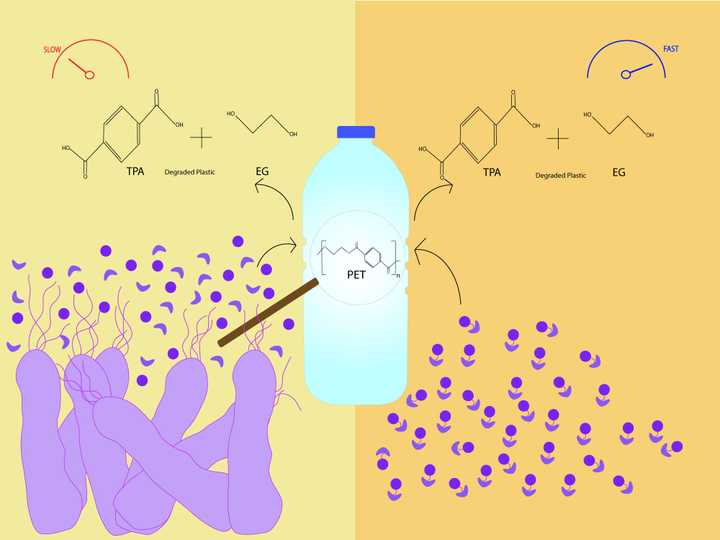
The bacteria Ideonella sakaiensis (purple) makes two enzymes - PETase and METase (circular and boomerang shaped purple molecules), which together act on polyethylene terephthalate (PET) to make the resultant products terephthalic acid (TPA) and ethylene glycol (EG). Right: The same enzymes when linked together to make a chimeric protein, degrade PET much faster. © Sunaina Rao.
Genetic engineering has made it possible for us to not only obtain the DNA sequence that codes for these enzymes, but also manipulate those sequences to construct better, more efficient versions of those enzymes.
To understand the functioning of any enzyme we first need to determine its 3D structure. This can give us an idea about specific regions, called domains, of the enzyme, that binds to the substrate (here plastic) before breaking it down. A group of scientists co-led by Dr. John E. McGeehan, from the University of Portsmouth and Dr. Gregg T. Beckham from the National Renewable Energy Laboratory (NREL), USA, have accomplished exactly this.
The team initially characterised the 3D structure of the newly discovered enzyme PETase from the bacteria Ideonella sakaiensis using high resolution X Ray crystallography. Following this, using the same technique, they further characterised the 3D structure of the second enzyme METase and ascertained important domains present in the enzyme. They show in their work that the two enzymes work in cooperation with each other, each requiring the others help in breaking down PET into its monomeric units - TPA and EG.
However what they hypothesised next was most fascinating. They wondered if PET could be degraded faster if the two enzymes were brought closer to each other. To this end, they engineered something called a chimeric protein, which contained both enzymes - PETase and METase, joined to each other via a linking protein sequence.To their pleasant surprise, this chimeric protein could degrade PET three times faster when compared to using the enzymes separately.
This remarkable discovery opens up many new avenues in plastic degradation. For one, there may be other related enzymes in nature that could degrade other plastic polymers. Which means we could engineer similar multi-enzyme systems to efficiently degrade other polymers as well as mixed polymer waste. The possibilities are innumerable and exciting.
However the problem is unfortunately far from solved. We still need to consider many factors. Firstly, is this enzyme system efficient and fast enough, keeping in mind the bulk of plastic waste out there? Since these studies have been performed in controlled laboratory conditions, what is its practical feasibility in the more unstable, natural environment. Furthermore, will it be scalable and cost effective?
For us to successfully resolve our plastic crisis, we need to take these and many more factors into consideration. In the years to come we may be able to fine tune our innovations to suit all requirements and hopefully save our planet, and ourselves from getting buried in an avalanche of plastic.